Ca Oh 2 Hcl
Ammonium chloride is an inorganic compound with the formula NH 4 Cl and a white crystalline salt that is highly soluble in water. Excess air 30 conversion of HCl 60.
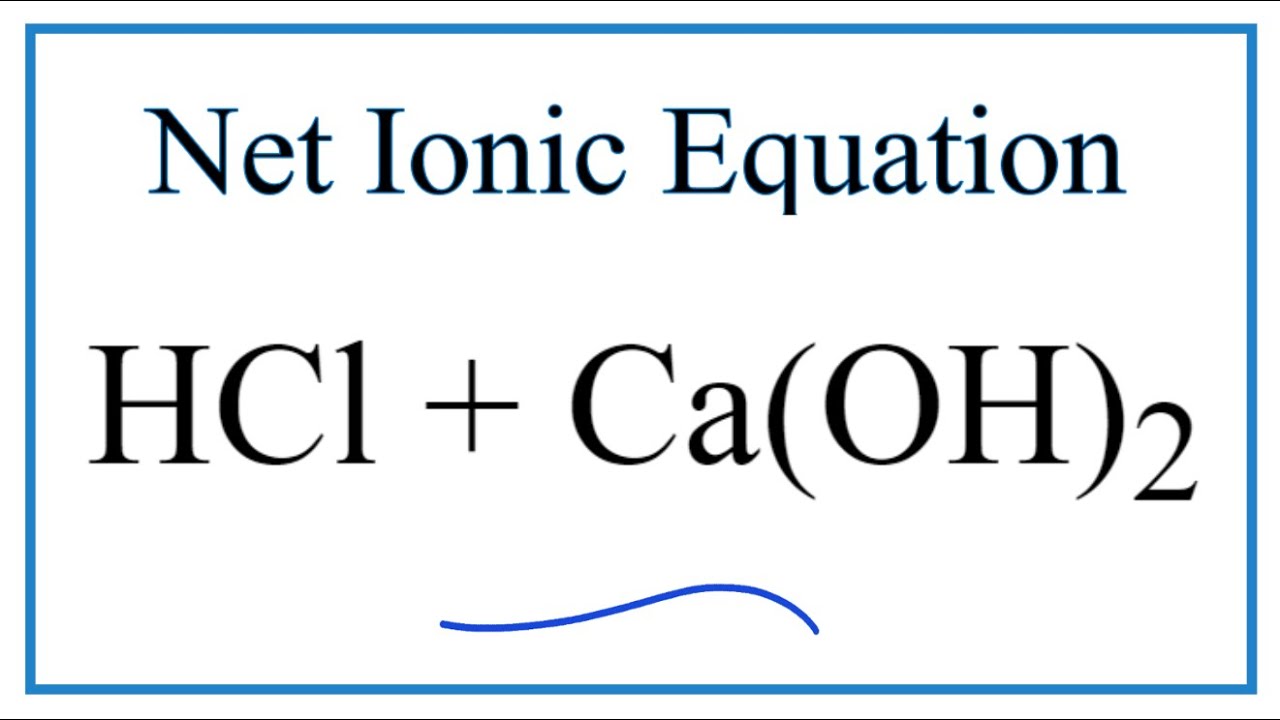
How To Write The Net Ionic Equation For Hcl Ca Oh 2 Cacl2 H2o Youtube
Nutrizoneca and any associates are not responsible for any statements or claims made by manufacturers about their products or accuracy of product.

. Clear All strong acid СаОН. Calcium hydroxide は化学式 CaOH 2 で表されるカルシウムの水酸化物 消石灰しょうせっかいとも呼ばれる固体はカルシウムイオンと水酸化物イオンからなるイオン結晶である 水溶液は石灰水懸濁液は石灰乳と呼ばれ共. As for strong bases - NaOH KOH LiOH CaOH 2 - pK b values read explanation in our FAQ section.
This is achieved with the. TABLE OF CONJUGATE ACID-BASE PAIRS Acid Base K a 25 oC HClO 4 ClO 4 H 2 SO 4 HSO 4 HCl Cl HNO 3 NO 3 H 3 O H 2 O H 2 CrO 4 HCrO 4 18 x 101 H 2 C 2 O 4 oxalic acid HC 2 O 4 590 x 102 H 2 SO 3 SO 2 aq H2 O HSO. Sabit oranlar yasası elementlerin birbirleri ile bileşik oluştururlarken belli oranda birleşmesine dayanan bir yasadır.
Clear All strong acid CaOH2 weak acid NH3 strong base HNO2 weak base KCI soluble salt H2CO3 insoluble salt. All pH neutralization reactions are exothermic and heat will be released. Create an equation for each element H Cl Na O where each term represents the number of atoms of the element in each reactant or product.
About us Feedback. 1 a 0b 1 c 0d Na. Hydroxide CaOH 2 potassium hydroxide KOH magnesium hydroxide MgOH 2 and ammonium hydroxide NH 4 OH.
H 2 CO 3 carbonic acid is the acid member of the pair because it can release H. Benefits support its short-term use. Calcium carbonate reacts with aqueous HCl to give C a C l 2 and C O 2 according to the reaction.
Am J Clin Nutr. HCL Cyber Security GRC services portfolio integrates business context intelligence threat data and cyber security insights. 1 a 1 b 0c 2 d Cl.
The chemical formula for hydrochloric acid is HCl and its molecular weight is 3647 gmol. In addition to mitigating threats and ensuring data integrity our solutions imparts agility to the enterprise. CaOH 2 2HCl CaCl 2 2H 2 0.
This system is important because two of its components are rigorously controlled by the body. Sanayi Devrimi sırasında sanayideki önemi keşfedilen asit önce. For the solutions with high ionic strength which are dubious in any case.
It is also found. Common side effects of buspirone include nausea headaches dizziness and difficulty concentrating. A double replacement reaction will occur if a formation of a precipitate gas or water takes place.
A 810 mL sample of 00500 M HBrO4 is titrated. 0a 1 b 0c 1 d. Solutions of ammonium chloride are mildly acidic.
In its naturally occurring mineralogic form it is known as sal ammoniacThe mineral is commonly formed on burning coal dumps from condensation of coal-derived gases. Santa Ana CA Cole JA Fordtran JS. When this happens its usually because the owner only shared it with a small group of people changed who can see it or its been deleted.
It is taken by mouth and it may take up to four weeks to have an effect. Bu orana ise sabit oranlar yasası denir. Conversely one mole of lime as CaOH 2 will neutralize two moles of HCl resulting in calcium chloride a salt.
Hidroklorik asit hidrojen ve klor elementlerinden oluşan oda sıcaklığı ve normal basınçta gaz hâlinde olan hidrojen klorürün sulu çözeltisine verilen ad. 134 Hydrochloric acid has an irritating pungent odor with an odor threshold of about 7 mgm 3. Elementler kimyasal olarak bir araya gelirken belli.
This is very important to improve stomach acid levels. Definitions and values of dissociation constants for weak and strong acids and bases - KOH NaOH HCl H2SO4 HClO4 HNO3 CaOH2 and other. 2 CO 3 H 2 O CO 2 The pair bicarbonate carbonic acid forms an important buffer system.
HCO 3-is the base member of the pair because it can accept H. Select two compounds above and this calculator will predict whether or not the reaction will occur in waterThis is simply based on the solubility chart of inorganic compounds. OH YEAH ONE BAR.
OH2 which does. N Put a drop of each of the above solutions on a watch-glass one by one and test with a drop of the indicators shown in Table 21. Create a System of Equations.
HCL has over two decades of experience in the cyber security services space. A 3000 mL sample of 010 M SrOH2 is titrated with 010 M HCl Therefore 1st option is right Q. Yüzyılda simyacı Cabir bin Hayyan tarafından keşfedildi ve sonrasında simya alanında kullanıldı.
0a 1 b 1 c 0d O. Solve For All Variables. If you have low HCL it is wise to have 1-2 protein shakes each day to enhance amino acid absorption and reduce stress on the GI system.
SAVAGE LINE LABS Creatine HCL 750 mg. Buspirone sold under the brand name Buspar among others is a medication primarily used to treat anxiety disorders particularly generalized anxiety disorder. N What change in colour did you observe with red litmus blue litmus.
A HCl b NaOH c NaCl d H 2 O. Halk arasında tuz ruhu olarak da bilinir. 13 Hydrochloric acid occurs as a colorless nonflammable aqueous solution or gas.
Bir bileşiği oluşturan elementlerin kütleleri arasında değişmeyen bir oran vardır. Is achlorhydria a cause of iron deficiency anemia. C a C O 3 s H C l a q C a C l 2 a q C O 2 g H 2 O l When 250 mL of 076 M HCl reacts with 1000 g of C a C O 3 calculate the number of moles of C a C l 2 formed.
In most practical applications the acid concentration is too low for the temperature rise to be a. Bu kanun 1799 yılında Joseph Proust tarafından bulundu.

Hcl Ca Oh 2 Cacl2 H2o Chemical Equation Balancer

Hcl Ca Oh 2 Cacl2 H2o Balanced Equation Hydrochloric Acid Calcium Hydroxide Balanced Equation Youtube
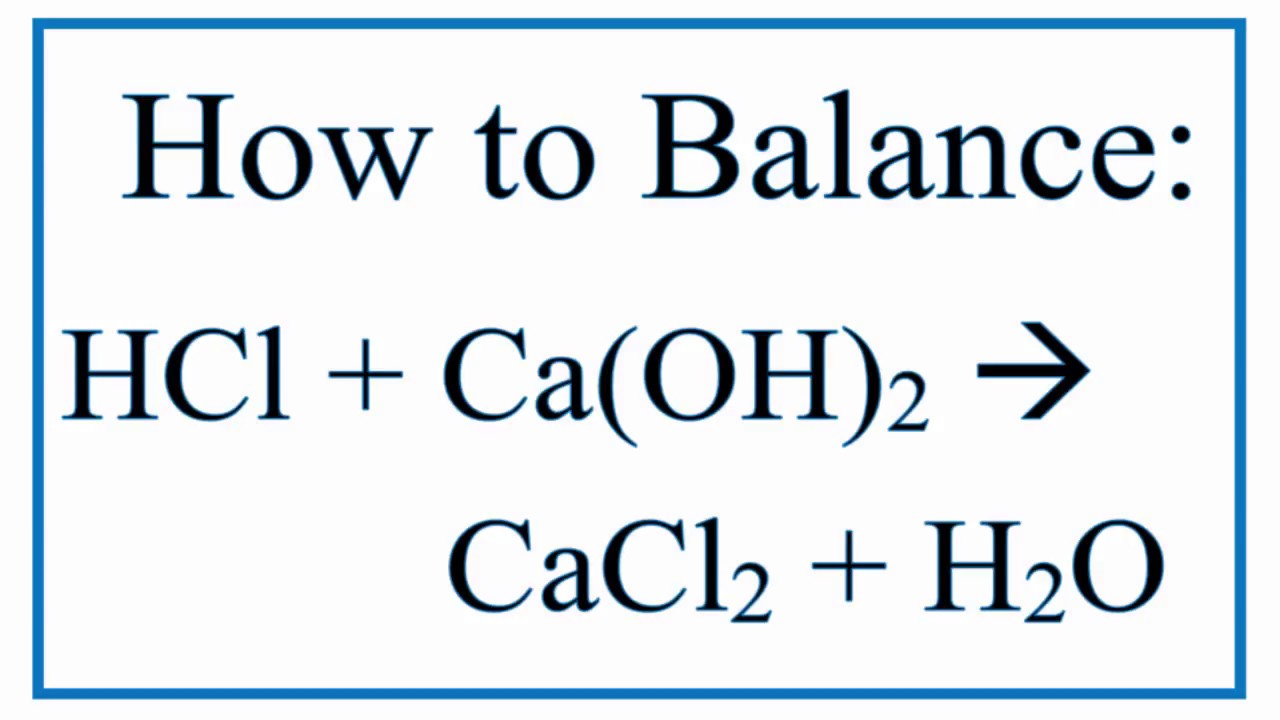
Balance Hcl Ca Oh 2 Cacl2 H2o Hydrochloric Acid And Calcium Hydroxide Youtube
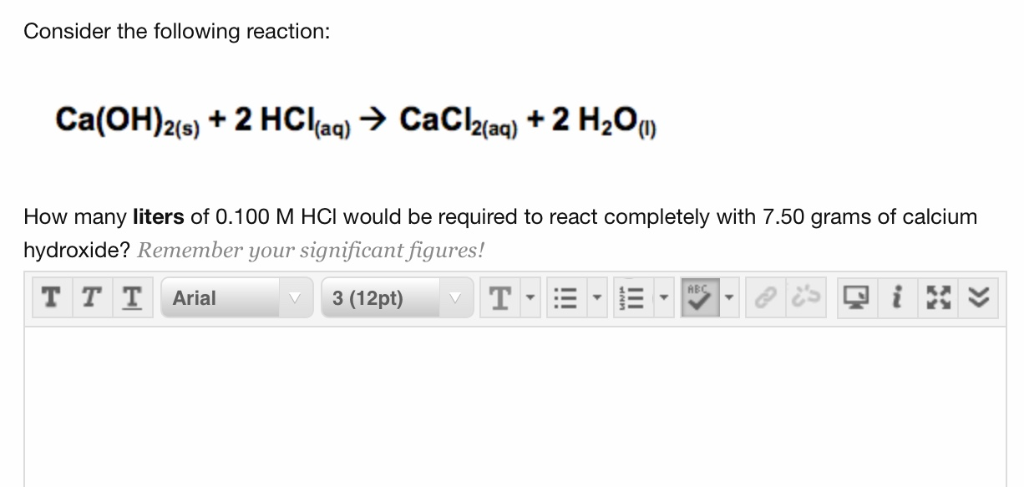
Solved Consider The Following Reaction Ca Oh 2 S 2 Chegg Com
Type Of Reaction For Hcl Ca Oh 2 Cacl2 H2o Youtube
0 Response to "Ca Oh 2 Hcl"
Post a Comment